Temperature and Dissolved Oxygen Levels
Click here to show/hide the data table
To display a different date, please select one from the list below:
Dissolved Oxygen
Oxygen is a natural element needed by all forms of life, including aquatic life. Most aquatic animals use oxygen dissolved in water. Oxygen primarily enters water via diffusion from surrounding air and from photosynthesis by aquatic plants. Dissolved oxygen is measured in units of milligrams/liter (mg/L) or as a percent of saturation (%).
Why do we measure it?
Oxygen is necessary for all living things and for many of the chemical processes that take place in water. The amount of dissolved oxygen needed by an aquatic organism depends on a variety of factors including the species, water temperature, and the species’ metabolic rate and overall health. Organisms typically have an optimum range in which they do best.
What affects it?
The temperature and salinity of water influence how much oxygen it can hold. Warm water holds less dissolved oxygen than cold water because the molecules are moving faster than in cold water and thereby allow oxygen to escape from the water. Freshwater can hold more dissolved oxygen than saltwater because saltwater has less space for oxygen molecules due to the sodium and chloride ions it contains. Therefore the warmer and saltier the water, the less dissolved oxygen it will contain.
Also, as mentioned previously, oxygen is added to water at the surface where gases in the atmosphere come into contact with it. Therefore, the movement of water from wind and waves can help oxygenate water. In addition, deeper water gets oxygen from the upper layers when mixing occurs. This mixing is aided when the density of water changes due to a change in water temperature.
Dissolved oxygen can also be influenced by humans. For instance, additional nutrients can enter a waterbody in runoff from lawns or farm fields, and cause a large increase in aquatic plant growth. While initially this may raise oxygen levels through photosynthesis, excess algal growth will consume oxygen on cloudy days and at night via respiration. Also, as all the plants die off, oxygen will be consumed by bacteria in the decomposition process
Temperature
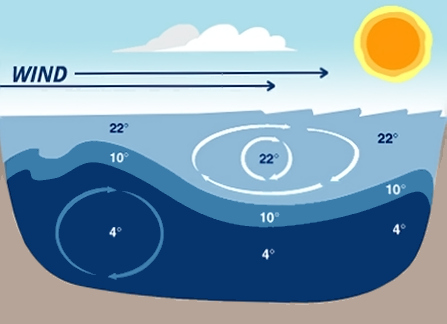
Temperature can vary depending on the time of year and the size and depth of a body of water.A unique phenomenon for lakes is the stratification of the water into layers due to changes in temperature at different depths. These layers occur because as water temperature changes so does its density. The stratification of a lake typically includes three general layers: the upper, warmer epilimnion, the middle metalimnion (which includes the thermocline), and the lower, colder hypolimnion. In the spring and fall, the lake will “turn-over” or mix when the water is very close to the same temperature from surface to the bottom. When the lake mixes the dissolved oxygen is mixed deeper into the lake which benefits the aquatic organisms.
Why do we measure it?
The temperature of water has a large impact on overall water quality as it directly influences many of the other parameters. It influences the amount of dissolved oxygen water can hold (colder water holds more). In addition, warmer water makes some substances more toxic for aquatic animals because the speed of chemical reactions usually increases at higher temperatures. Temperature directly influences aquatic life by impacting the rate of metabolism, photosynthesis, growth, decay, etc. Every aquatic animal has an optimum temperature range that is best for its health.
It is also important to be aware of the negative impact climate change could have on water quality. For example, a potential increase in severe rain events could lead to elevated concentrations of pollutants and nutrients entering a waterway from runoff. The increase of nutrients, such as nitrogen and phosphorus, could lead to more algal blooms, which in turn could result in a decrease of dissolved oxygen.
What affects it?
Temperature naturally varies at different depths and by season. Also, riparian vegetation produces shade, which can cool water. Conversely, when the vegetation is removed, more sunlight can penetrate and warm up the water. In addition, erosion can raise water temperature by increasing suspended particles, which absorb the sun’s heat.